Impact of Biofeedback and Personalized Nutrition on Glycemic Control, Multi-Omics Markers and Mitochondrial Health in Early Dysglycemic Individuals: A Stratified Randomized Controlled Trial.
Project Status
Proposal submitted for intermediary grant funding; under review by ICMR (Indian Council of Medical Research). Pre-award preparatory work completed (design, sample-size model, assessment packages, intervention staging, analysis plan).
Role
Project Lead and Coauthor
Led the design and development of the project proposal and analysis framework for evaluating biofeedback tools and personalized dietary interventions. Coordinated a multidisciplinary team of doctors, researchers, and data analysts to deliver a complete project proposal, including study design, budget breakdown, analysis plan, and implementation steps.
Research Proposal Presentation
Summary
Rational: India is facing a growing metabolic health crisis, with 23.7 crore individuals classified as dysglycemic. Of these, 57.38% are in the prediabetic stage, as defined by ICMR/WHO criteria. While lifestyle interventions are known to delay diabetes progression, there is limited evidence in the Indian context on the use of continuous biofeedback tools to enhance the effectiveness of such interventions.
Novelty: This study uniquely integrates three continuous biofeedback tools continuous glucose monitors (CGMs), body composition analyzers, and smart calorie-tracking apps with personalized dietary interventions. It combines real-time feedback with in-depth biological profiling to explore not only glycemic improvements but also the reversibility of underlying metabolic dysfunctions.
Objectives: To assess the effectiveness of a tech-enabled, personalized dietary intervention in reversing or improving glycemic control in individuals with prediabetes, and to examine the mechanistic changes using multi-omics analysis.
Methods: A stratified randomized controlled trial will be conducted. Intervention participants will receive tailored dietary guidance supported by CGMs, body composition metrics, and calorie-tracking apps. Both intervention and control groups will undergo multi-omics profiling including metabolomics, lipidomics, and mitochondrial assessments along with gut microbiome and genetic analysis.
Expected Outcome: The study aims to demonstrate the efficacy of combining biofeedback tools with nutrition counseling in improving glycemic markers and reversing metabolic dysfunction. Findings will support the development of scalable, tech-integrated preventive care models for early-stage dysglycemia in India.
Why Does this Matter
Early dysglycemia is heterogeneous; response to “one-size-fits-all” advice varies. By combining real-time physiology (CGM, BIA) with personalized nutrition tracked via a smart food-logging app and with periodic structured multi-omics assessments of the stratified randomized groups, the study aims to improve glycemic control and map potential biological indicators that possesses connections with the biofeedback tools used, to benefit Indian citizens of having a way to self driven and easily track their progress in a more personalized and realistic manner based on their body physiology and chemicoly reactions, giving them power to control track and assess their health individually within expensive and delayed treatments/ interventions.
Hypothesis and Research Question
Population - Adults with dysglycemic levels (prediabetes) recruited from a private hospital in Sector 51, Gurgaon.
Intervention - Continuous biofeedback tools (BIA scales, CGMs, AI-based dietary tracking) combined with MRI/MRS scans and personalized dietary intervention strategies.
Comparison - Standard prediabetes management per ICMR guidelines (educational materials, six monthly lab and MRI/MRS assessments).
Outcome - Improved glycemic control, MRI/MRS parameters, and favourable shifts in multi-omics and mitochondrial health.
Research Question - Can integrating continuous biofeedback devices with personalized dietary management strategies enhance glycemic control, improve multi-omics markers and mitochondrial health indicators related to glycemic control, and reduce diabetes progression more effectively than standard ICMR guidelines?
Study Design
Study Type and Recruitment
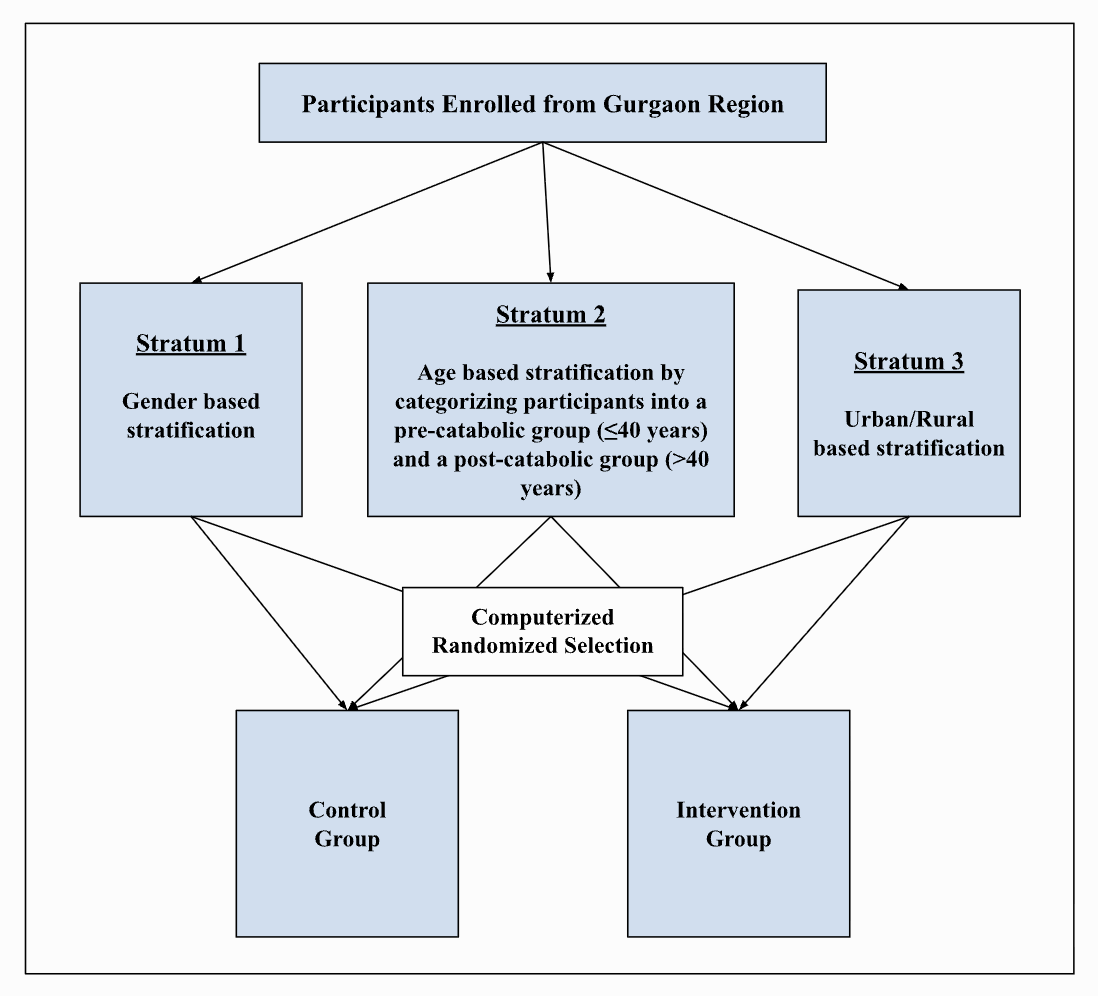
This is a prospective, stratified randomized controlled trial targeting adults with prediabetes. Participants will be recruited through preventive health checkups, corporate employee screenings, and targeted outreach programs. Eligibility will be confirmed with blood and stool tests, including hemogram, HbA1c, fasting glucose tolerance test, and metabolomics screening. Following describes the stratification strategy for the study.
Study Groups
Control Group: Follows standard ICMR prediabetes guidelines (balanced diet, physical activity, sleep hygiene) with follow-up assessments every six months.
Intervention Group: Follows the same guidelines plus staged integration of biofeedback and dietary tools:
Bioelectrical Impedance Analysis (BIA) scale
Continuous Glucose Monitors (CGM)
Smart calorie-tracking application
Three structured dietary consultations
Assessment Package Details
Baseline Package
Anthropometrics: weight, height, waist circumference, blood pressure
Body composition (BIA + MRI for fat distribution)
Blood tests: HbA1c, fasting glucose, insulin, hemogram
Stool tests: ova and cysts
Multi-omics profiling: metabolomics, lipidomics, genomics (blood), microbiome (stool)
Lifestyle and demographic history
6-Monthly Package
Repeats all baseline health assessments
Reinforcement of ICMR guidelines
Multi-omics repeated only at endpoint (genomics done once)
Intervention Package (intervention group only)
BIA scale for continuous body composition tracking
Two CGMs (for baseline monitoring + post-diet plan validation)
Smart calorie tracker for real-time food logging
Three diet consultations (baseline, mid, final)
Intervention Group Setup Stages
Stage 1: Receive BIA scale + baseline assessments
Stage 2: Weekly monitoring & compliance checks
Stage 3: First CGM + Smart calorie tracker for 15 days (normal diet logging)
Stage 4: Personalized dietary plan created using CGM + food log data
Stage 5: Second CGM + diet plan validation
Stage 6: Return to BIA scale tracking + ongoing monitoring
Stage 7: Six-monthly follow-ups until study completion (2 years)
Outcomes Expected for the Study
Primary Outcomes
Progression or regression of glycemic status (prediabetes → normal / diabetes)
Changes in obesity category (based on BMI and body composition)
Secondary Outcomes
Associations between biofeedback data (CGM, BIA, calorie logs) and multi-omics profiles
Changes in body composition, metabolic markers, and lifestyle adherence
Insights into the biological impact of using continuous, consumer-friendly health monitoring tools
Sample Size and Recruitment Strategy
This study will use a prospective, stratified randomized controlled trial (RCT) with one control and one intervention group, observed in parallel with no crossover. Participants will be recruited from preventive health checkups (urban), employee wellness programs, and rural outreach initiatives.
To minimize bias and ensure balance, we will use stratified randomization based on three key factors:
Age: ≤40 years (pre-catabolic) vs. >40 years (post-catabolic)
Gender: male vs. female
Residency: urban vs. rural
These variables are important because they influence glycemic control, metabolic health, and response to interventions. This creates 8 strata (2 × 2 × 2), ensuring equal representation across groups.
Sample Size Estimation
Using a parallel-group design with α = 0.05, 80% power, and effect size from prior literature, the base requirement was 63 participants per group (126 total).
To maintain at least 10 participants per group in each stratum, the adjusted total was 160.
With a 10% attrition buffer, the final target is 178 participants (89 per group).
Feasibility
The medical center diagnoses ~50–70 new prediabetes cases per month. Assuming one in three consents, recruitment should reach 15–20 participants per month, allowing completion within 8–10 months.
This structured approach ensures statistical validity, balanced groups, and results that are generalizable across diverse prediabetic populations.
Statistical Analysis Strategy
Longitudinal Analysis (Tracking Health Over Time)
Key outcomes fasting glucose, HbA1c, and body composition will be measured at baseline, midpoint, and endpoint.
We will use linear mixed-effects models (LMMs), a method designed for repeated measures on the same individuals. This accounts for both fixed effects (like time, group, age, sex, BMI) and random effects (differences between individuals).
A time × group interaction will test whether the intervention leads to greater improvements compared to standard care.
If some data are missing, the model can handle it under the “Missing At Random” (MAR) assumption.
For variables that are not normally distributed, data transformations (e.g., log-scale) or non-parametric tests will be applied.
Statistical significance will be set at p < 0.05 (meaning less than 5% chance the result is random).
Translational Analysis (Understanding Biological Mechanisms)
To explore why health changes occur, we will analyze multi-omics data:
Microbiome: Gut bacterial diversity (alpha and beta diversity) and changes in specific taxa will be tested with methods like DESeq2 or ANCOM. Associations with health outcomes will be explored using mixed-effects models.
Metabolomics & Lipidomics: Both single-variable tests (with False Discovery Rate [FDR] correction) and broader pattern-recognition techniques (e.g., PCA, PLS-DA) will be used. Correlation networks and machine learning models (e.g., random forest) may help identify predictive biomarkers.
Genomics: Gene–diet interactions will be examined using regression models with interaction terms. Key SNPs linked to glycemic traits and insulin resistance will be assessed for their role in intervention response.
Multiple testing will be corrected using the Benjamini–Hochberg FDR method to reduce false positives.
Connecting the Data - Integrated Interpretation
We will combine clinical results and omics data to get a complete picture of how biofeedback tools and personalized diets affect blood sugar and weight in prediabetes. Pathway and systems biology tools (e.g., KEGG, Reactome) will help link the different data layers.
Actions following the completion of project
To validate reproducibility across varied populations, a multicentric follow-up will be launched. In parallel, pilot implementation will begin in collaboration with hospitals and public health programs, with systems biology assessment evaluating integration and responsiveness of multi-dimensional data (clinical, behavioral, metabolic) in real-world settings. Policy briefs will be developed, and stakeholder dialogues with ICMR and the Ministry of Health conducted to advocate for inclusion of biofeedback-driven, personalized interventions in national diabetes prevention frameworks. These efforts aim to catalyze clinical adoption and support evidence-based updates to prediabetes management guidelines.